Nickel-catalyzed direct formation of the C–S bonds of aryl sulfides from arylsulfonyl chlorides and aryl iodides using Mn as a reducing agent †
Abstract
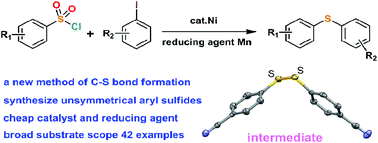
Various unsymmetrical aryl sulfides were synthesized by nickel-catalyzed C–S bond formation in good to excellent yields. The reactions employed arylsulfonyl chlorides as an aryl thiol source and Mn dust as a reducing agent. The scope and versatility of the method have been successfully demonstrated with 42 examples. Mechanistic studies revealed the existence of an intermediate disulfide substance. A catalytic cycle was proposed including a three-step reduction by Mn for the achievement of the reaction, and Ni(0) and Ni(I) species were supposed to be involved in the reaction mechanism.

 Please wait while we load your content...
Please wait while we load your content...