Manganese-catalyzed ring-opening chlorination of cyclobutanols: regiospecific synthesis of γ-chloroketones†
Abstract
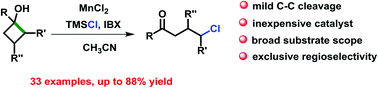
We disclose an efficient manganese-catalyzed ring-opening chlorination of cyclobutanols. This reaction has a broad substrate scope, affording a variety of γ-chloro alkyl ketones and medium-sized benzocyclic chlorides in synthetically useful yields and unique regioselectivities. The merits including mild reaction conditions and employment of inexpensive catalysts and reagents make it a practical approach for the production of γ-chlorinated ketones.

 Please wait while we load your content...
Please wait while we load your content...