Sulfonyl radical-enabled 6-endo-trig cyclization for regiospecific synthesis of unsymmetrical diaryl sulfones†
Abstract
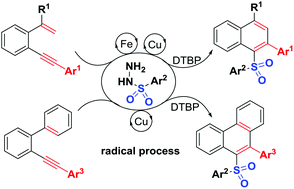
A new sulfonyl radical-triggered 6-endo-trig cyclization of unactivated 1,5-enynes under catalytic oxidation conditions for flexible synthesis of unprecedented unsymmetrical diaryl sulfones was established. This transformation could also proceed smoothly using 2-arylalkynyl-1,1′-biphenyls, delivering new sulfonylated phenanthrenes. The synthetic utility of these transformations results in subsequent C–S and C–C bond-forming reactions to effectively build up functional sulfones with potential significance.


 Please wait while we load your content...
Please wait while we load your content...