A cross-coupling-annulation cascade from peri-dibromonaphthalimide to pseudo-rylene bisimides†
Abstract
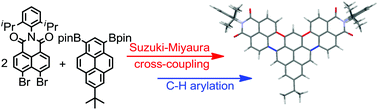
Herein we report a synthetic method to annulate naphthalimide moieties at different positions of a pyrene core by a cascade reaction involving palladium-mediated Suzuki–Miyaura cross-coupling and direct C–H arylation affording a new class of rylene bisimides, which we term as pseudo-rylene bisimides. The Pd-mediated reactions of peri-dibromonaphthalimide and pyrene boronic acid esters lead exclusively to six-membered ring annulation by a cascade C–C bond formation, for which a mechanistic rationale is proposed. Depending on the molecular geometry of the new pseudo-rylene bisimides, their optical properties resemble those of zethrene or terrylene bisimides, or polycyclic aromatic hydrocarbons like coronenes. The structural integrity of this new type of pseudo-rylene bisimides was confirmed by single crystal X-ray analysis.
- This article is part of the themed collection: Novel π-electron molecular scaffolds


 Please wait while we load your content...
Please wait while we load your content...