Iodosobenzene-mediated direct and efficient oxidation of β-dicarbonyls to vicinal tricarbonyls catalyzed by iron(iii) salts†
Abstract
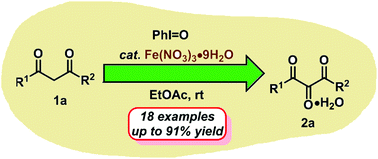
Iodosobenzene was found to be effective in the direct oxidation of β-dicarbonyls to vicinal tricarbonyl compounds catalyzed by Fe(NO3)·9H2O under mild and environmentally friendly conditions. Also, the present protocol was applied to the one-pot synthesis of heterocyclic compounds via vicinal tricarbonyls as the reactive intermediates. As for the reaction pathway, a plausible mechanism involving iodonium ylide, reactive carbene species and an α-hydroxylated intermediate was proposed.

 Please wait while we load your content...
Please wait while we load your content...