LDA-mediated synthesis of ortho-cyanated diarylmethanes by reaction of fluoroarene with arylacetonitrile at room temperature†
Abstract
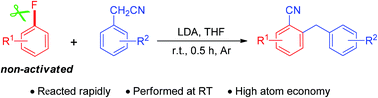
An efficient and facile method for the synthesis of ortho-cyanated diarylmethane by reaction of fluoroarene with arylacetonitrile in the presence of LDA (lithium diisopropylamide) at room temperature was developed. The reaction of arylacetonitrile with electron-rich fluoroarene involves the formation of an aryne intermediate.

 Please wait while we load your content...
Please wait while we load your content...