Stereo- and regio-selective synthesis of 3′-C-substituted-(N)-methanocarba adenosines as potential anticancer agents†
Abstract
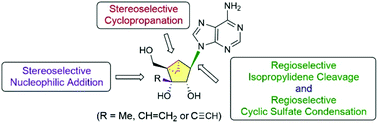
Stereo- and regio-selective synthesis of 3′-C-substituted-(N)-methanocarba adenosines 3a–c as potential anticancer agents was achieved by employing stereoselective cyclopropanation, stereoselective nucleophilic addition, regioselective isopropylidene cleavage and regioselective base condensation of cyclic sulfate as key steps.

 Please wait while we load your content...
Please wait while we load your content...