Diastereoselective synthesis of 2,5-disubstituted-3-hydroxy-tetrahydrofurans through a counterion-directed Tsuji–Trost reaction†
Abstract
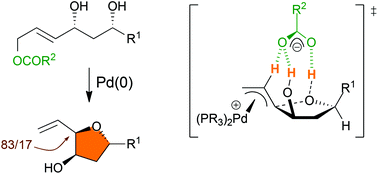
Tsuji–Trost Counterion Directed Catalysis (CDC) allows the diastereoselective synthesis of 5-alkyl-3-hydroxy-2-vinyl tetrahydrofurans in high yields using easily accessible 4,6-dihydroxyalk-2-en-1-yl esters as substrates. The mechanism of this reaction has been investigated by relying on DFT predictions and experimental confirmations based on deuterium labeling and modification of the pKa of counterions which demonstrates that the favored transition state is tightly organized through the formation of a network of H-bonds.

 Please wait while we load your content...
Please wait while we load your content...