On the scope of oxidation of tertiary amines: Meisenheimer rearrangements versus Cope elimination in 2-(cyanoethyl)-2-azanorbornanes†
Abstract
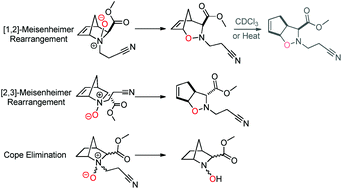
In this work, rearrangement reactions subsequent to the oxidation of tertiary amines were studied in 2-(cyanoethyl)-2-azanorbornane/ene systems. [1,2]- and [2,3]-Meisenheimer rearrangements, as well as the Cope elimination reaction, were observed with virtually complete selectivity. It was found that 2-(cyanoethyl)-2-azanorbornanes afford N-hydroxylamines through Cope elimination reactions and 2-(cyanoethyl)-2-azanorbornenes are prone to Meisenheimer rearrangements. In addition, the endo/exo configuration of 2-azanorbornenes plays a key role in the Meisenheimer rearrangement outcome. All the synthesized compounds were fully characterized by NMR spectroscopy and HRMS.


 Please wait while we load your content...
Please wait while we load your content...