A new method to access triazole-fused spiro-guanidines from the reaction of isothiocyanates tethered N-sulfonyl-1,2,3-triazoles and amines†
Abstract
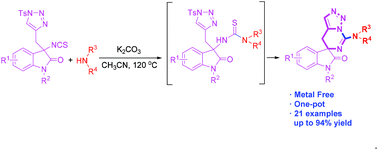
A novel method to access triazole-fused spiro-guanidine from the reaction of isothiocyanate tethered N-sulfonyl-1,2,3-triazoles and amine has been developed. The reactions proceeded through a two-component tandem reaction, including nucleophilic addition, base-assisted intramolecular (or intermolecular) nucleophilic substitution and subsequent intramolecular nucleophilic addition–elimination process. A wide range of protected oxindole skeletons and amines are well-tolerated.

 Please wait while we load your content...
Please wait while we load your content...