Visible light-mediated intramolecular C–H arylation of diazonium salts of N-(2-aminoaryl)benzoimines: a facile synthesis of 6-arylphenanthridines†
Abstract
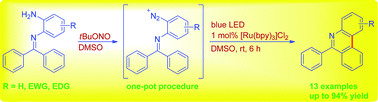
Utilizing [Ru(bpy)3]Cl2 as a photoredox catalyst and a blue LED as an irradiation source, moderate to good yields of 6-arylphenanthridines were obtained from aryl diazonium salts in situ formed from the reactions of N-(2-aminoaryl)benzoimines and tert-butyl nitrite (tBuONO). Although few visible light-mediated protocols are available for the synthesis of 6-arylphenanthridines, this work provides a new method to access them readily from inexpensive and environmentally benign starting materials.

 Please wait while we load your content...
Please wait while we load your content...