Fe-Catalyzed reductive NO-bond cleavage – a route to the diastereoselective 1,4-aminohydroxylation of 1,3-dienes†
Abstract
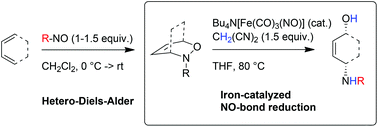
The low-valent Fe-complex Bu4N[Fe(CO)3(NO)] (TBA[Fe]) catalyzes the reductive cleavage of N–O-bonds in oxazines under salt-free conditions using malononitrile as reductant. The mild reduction conditions allow the development of a sequence of [4 + 2]-cycloaddition/reductive cleavage, a process that can be considered as an efficient way for the diastereoselective 1,4-aminohydroxylation of 1,3-dienes.
- This article is part of the themed collection: Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...