Diastereo- and enantioselective construction of cyclohexanone-fused spirospyrazolones containing four consecutive stereocenters through asymmetric sequential reactions†
Abstract
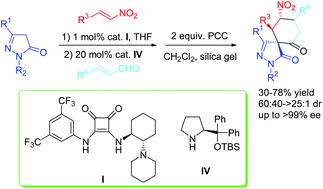
The diastereo- and enantioselective synthesis of cyclohexanone-fused spirospyrazolones containing four consecutive chiral centers has been successfully developed through an asymmetric Michael/Michael/aldol cascade reaction catalyzed by the combination of a bifunctional squaramide and diphenylprolinol silyl ether, followed by a sequential oxidation with pyridinium chlorochromate. This protocol affords cyclohexanone-fused spirospyrazolones in moderate to high yields, moderate to good diastereoselectivities and perfect enantioselectivities.
- This article is part of the themed collection: Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...