Non-stabilized enolates – versatile nucleophiles in transition metal-catalysed allylic alkylations†
Abstract
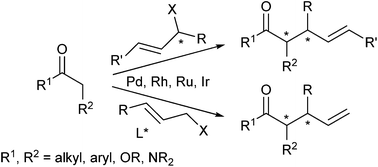
While in the early days of palladium-catalyzed allylic alkylations mainly stabilized carbanions, such as malonates, have been used as standard nucleophiles, during the last 10–15 years also non stabilized enolates became more and more popular. Efficient protocols have been developed allowing their highly regio- as well as stereoselective allylations. In addition, catalysts based on other transition metals enlarge the synthetic potential of these valuable transformations, since each metal (complex) shows its own, typical reaction behaviour. This review will cover the new developments in this fast developing field.
- This article is part of the themed collections: Celebrating the 75th Birthday of Professor Barry Trost and 2016 Organic Chemistry Frontiers Review-type Articles

 Please wait while we load your content...
Please wait while we load your content...