A zinc-catalyzed oxidative reaction of ynamides with phenols and thiophenols: highly site-selective synthesis of versatile α-aryloxy amides and α-arylthio amides†
Abstract
A zinc-catalyzed oxidative reaction of ynamides with phenols and thiophenols under mild reaction conditions has been developed, which provides various α-aryloxy amides and α-arylthio amides in moderate to good yields, respectively. Importantly, high chemoselectivity is achieved by such a non-noble metal-catalyzed alkyne oxidation.
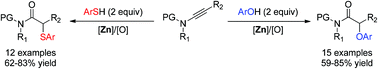

 Please wait while we load your content...
Please wait while we load your content...