Generation of benzosultams via trifluoromethylation of 2-ethynylbenzenesulfonamide under visible light†
Abstract
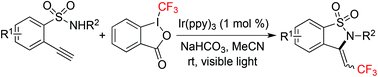
Under visible light irradiation, 2-ethynylbenzenesulfonamides react with Togni's reagent in the presence of a photocatalyst leading to 3-(2,2,2-trifluoroethylidene)-2,3-dihydrobenzo[d]isothiazole 1,1-dioxides in good yields. This transformation proceeds efficiently at room temperature through a photo-initiated trifluoromethylation. The subsequent C–N bond formation produces the corresponding benzosultams.

 Please wait while we load your content...
Please wait while we load your content...