CuBr2-promoted cyclization and bromination of arene–alkynes: C–Br bond formation via reductive elimination of Cu(iii) species†
Abstract
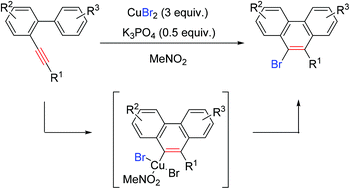
A CuBr2-promoted cyclization and bromination of arene–alkyne substrates has been developed, affording 9-bromophenanthrene derivatives highly efficiently. This reaction provides a novel and efficient protocol for C–Br bond formation from an inorganic bromine source via the reductive elimination of the Cu(III) species.

 Please wait while we load your content...
Please wait while we load your content...