A general route to sulfones via insertion of sulfur dioxide promoted by cobalt oxide†
Abstract
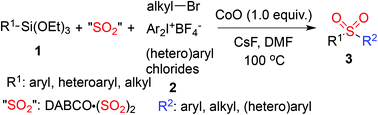
A cobalt-promoted coupling reaction of triethoxysilanes, sulfur dioxide, and electrophiles is developed. Benzylic, allylic or alkyl bromides are all efficient as electrophiles in this transformation. Moreover, the reactions with other electrophilic partners including iodonium salts and electron-poor (hetero)aryl chlorides proceed smoothly as well under the standard conditions. The sulfinate intermediates are generated in situ from the reaction of triethoxysilanes and sulfur dioxide under cobalt-promoted conditions, which subsequently combine with various electrophiles to produce the corresponding sulfones in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...