Tartrate-derived iminophosphorane catalyzed asymmetric hydroxymethylation of 3-substituted oxindoles with paraformaldehyde†
Abstract
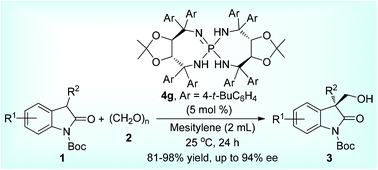
The enantioselective synthesis of 3-hydroxymethyl-2-oxindoles was achieved through organocatalysis by a tartrate-derived chiral iminophosphorane in 81–98% yields and up to 94% ee under mild conditions. Of note is that readily available and easily usable paraformaldehyde was employed as a hydroxymethylation C1 unit in the reaction.

 Please wait while we load your content...
Please wait while we load your content...