A general route to fluorinated 3,3-disubstituted 2-oxindoles via a photoinduced radical cyclization of N-arylacrylamides under catalyst-free conditions†
Abstract
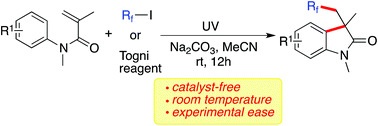
A catalyst-free radical cyclization of N-arylacrylamides with fluorinated alkyl iodides or the Togni reagent enabled by photoenergy is presented for the first time. Under ultraviolet irradiation, the generation of fluorinated 3,3-disubstituted 2-oxindoles proceeds smoothly without any metals or photoredox catalysts. The broad reaction scope is demonstrated with good functional group tolerance. During the process, fluorinated alkyl groups can be easily incorporated.

 Please wait while we load your content...
Please wait while we load your content...