A versatile access to vicinal diamine motifs by highly anti-selective asymmetric vinylogous Mannich reactions: an efficient total synthesis of (+)-absouline†
Abstract
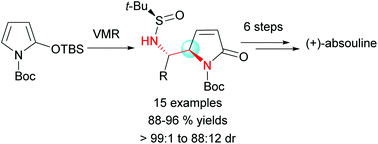
We report the asymmetric vinylogous Mannich reactions (VMRs) of N-Boc-2-tert-(butyldimethylsilyloxy)pyrrole (TBSOP) with N-tert-butanesulfinylimines. The reaction is highly anti-diastereoselective and shows good generality for the direct construction of a variety of vicinal anti-diamine motifs that are found in a number of biologically active alkaloids and medicinal agents. A VMR adduct was elaborated in six steps into the 1-aminopyrrolizidine alkaloid (+)-absouline, which constitutes the second and also the most efficient total synthesis of the natural enantiomer of the title alkaloid.

 Please wait while we load your content...
Please wait while we load your content...