Rhodium-catalyzed oxidative coupling of N-acyl anilines with alkynes using an acylamino moiety as the traceless directing group†
Abstract
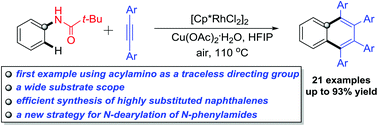
A rhodium-catalyzed oxidative annulation of N-acyl anilines with alkynes was developed by using the acylamino group as a traceless directing group for the first time. Various N-acyl anilines and para- or meta-substituted diphenylacetylenes were well tolerated, and a series of 1,2,3,4-tetrasubstituted naphthalenes were readily synthesized in good to excellent yields. Meanwhile, this method also provides a new strategy for the N-dearylation of N-phenylamides.

 Please wait while we load your content...
Please wait while we load your content...