Cu-catalyzed hydrofluoroacetylation of alkynes or alkynyl carboxylic acids leading highly stereoselectively to fluoroacetylated alkenes†
Abstract
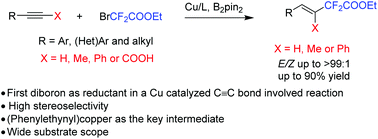
A copper-catalyzed hydrofluoroacetylation of arylacetylenes or alkynyl carboxylic acids with bromofluoroacetates was developed. The reaction shows excellent stereoselectivity to afford fluoroacetylated alkenes in good to excellent yields with a broad substrate scope. A preliminary mechanism study suggested that a radical pathway was involved in the reaction and (phenylethynyl)copper might be the key intermediate for the reaction. B2pin2 also plays an indispensable role in this novel hydrofluoroacetylation reaction.

 Please wait while we load your content...
Please wait while we load your content...