Cu-catalyzed sequential C–N bond formations: expeditious synthesis of tetracyclic indoloindol-3-ones†
Abstract
The tetracyclic indoloindol-3-one core has been forged from easily accessible 2,2′-bis-bromochalcones employing a reaction cascade comprising Cu-catalyzed SNAr with azide; nitrene C–H insertion and intramolecular Ullmann reaction with all three C–N bond formations in one-go.
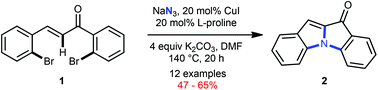

 Please wait while we load your content...
Please wait while we load your content...