Cu3Ru6Sb8—a new ternary antimonide with a new structure type†
Abstract
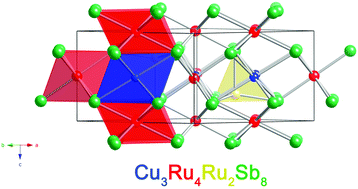
The new ternary transition metal antimonide, Cu3Ru6Sb8, has been synthesized by a solid-state reaction of the elements at 1023 K. Its crystal structure has been established by single-crystal X-ray diffraction methods. It crystallizes in a new structure type in the trigonal crystal system (Pearson index hP17) in space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1. The asymmetric unit of this structure contains six crystallographically independent sites: one Cu (site symmetry .2/m.), three Ru (Ru1 (.2/m.), Ru2 (3m.), and Ru3 (
m1. The asymmetric unit of this structure contains six crystallographically independent sites: one Cu (site symmetry .2/m.), three Ru (Ru1 (.2/m.), Ru2 (3m.), and Ru3 (![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m.)), and two Sb sites (Sb1 (.m.) and Sb2 (3m.)). Two of the Ru atoms and the Cu atom are coordinated to six Sb atoms in a distorted octahedral fashion; the third Ru atom is found in a trigonal bipyramidal environment of five Sb atoms. The structure can be viewed as a hexagonal closed-packed array of Sb atoms, with Ru and Cu atoms in the interstices, representing a lattice that is an ordered variant of the NiAs structure. Electronic structure calculations provide insight into the chemical bonding in this transition metal antimonide. From magnetic and resistivity measurements on polycrystalline material, the compound is metallic and exhibits magnetic response that shows an effective moment smaller than any free-ion values, suggestive of weak itinerant magnetism.
m.)), and two Sb sites (Sb1 (.m.) and Sb2 (3m.)). Two of the Ru atoms and the Cu atom are coordinated to six Sb atoms in a distorted octahedral fashion; the third Ru atom is found in a trigonal bipyramidal environment of five Sb atoms. The structure can be viewed as a hexagonal closed-packed array of Sb atoms, with Ru and Cu atoms in the interstices, representing a lattice that is an ordered variant of the NiAs structure. Electronic structure calculations provide insight into the chemical bonding in this transition metal antimonide. From magnetic and resistivity measurements on polycrystalline material, the compound is metallic and exhibits magnetic response that shows an effective moment smaller than any free-ion values, suggestive of weak itinerant magnetism.
- This article is part of the themed collection: In honour of Mercouri G. Kanatzidis for his contributions to Inorganic Chemistry for over 30 years


 Please wait while we load your content...
Please wait while we load your content...