Radical pathways and O2 participation in benzyl alcohol oxidation, and catechol and o-aminophenol oxidase activity studies with novel zinc complexes: an experimental and theoretical investigation†
Abstract
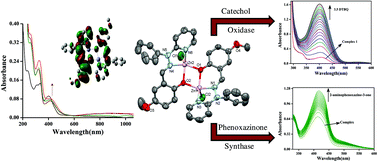
Diphenoxo bridged dinuclear zinc complexes [Zn2(OMe-Phimp)2(Cl)2] (1) (OMe-PhimpH = (E)-4-methoxy-2-((2-phenyl-2-(pyridin-2-yl)hydrazono)methyl)phenol), [Zn2(Me-Phimp)2(Cl)2] (2) (Me-PhimpH = (E)-4-methyl-2-((2-phenyl-2-(pyridin-2-yl)hydrazono)methyl)phenol), [Zn2(N-Phimp)2(Cl)2]·CH3CN (3·CH3CN) (N-PhimpH = (E)-1-((2-phenyl-2-(pyridin-2-yl)hydrazono)methyl)naphthalen-2-ol) and [Zn2(Phimp)2(Cl)2] (4) (PhimpH = (E)-2-((2-phenyl-2-(pyridin-2-yl)hydrazono)methyl)phenol) were synthesized and spectroscopically characterized. The molecular structures of 1 and 3·CH3CN were determined using X-ray crystallography. DFT and TD-DFT calculations were performed to optimize the molecular geometry, interpret the spectroscopic results and investigate the contribution of the ligands to the redox properties of the complexes. Phenoxyl radical complexes were generated in solution via chemical oxidation using ceric ammonium nitrate (CAN) and the redox properties were examined through cyclic voltammetric measurements. All the dinuclear zinc complexes were found to be highly active in catalyzing the oxidation of the primary alcohols 3,5-di-tert-butylcatechol and o-aminophenol. The catalytic activity was found to be in the order of complex 1 > complex 2 > complex 4 > complex 3 for both calechol oxidation and o-aminophenol oxidation. An EPR experiment clearly hinted at the generation of a radical during the oxidation of catechol and o-aminophenol. Reaction models for these processes were proposed and theoretical studies were performed to support the proposed mechanism. ESI-MS spectra clearly indicate the formation of a catalyst–substrate adduct by removal of one Cl− ion.

 Please wait while we load your content...
Please wait while we load your content...