Harnessing uranyl oxo atoms via halogen bonding interactions in molecular uranyl materials featuring 2,5-diiodobenzoic acid and N-donor capping ligands†
Abstract
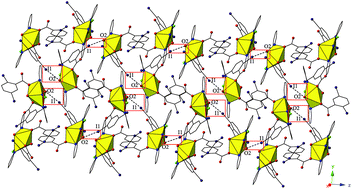
The syntheses and crystal structures of five new compounds containing the UO22+ cation, 2,5-diiodobenzoic acid, and a chelating N-donor (2,2′-bipyridine (bipy) (1), 1,10-phenanthroline (phen) (2 and 3), 2,2′:6′,2′′-terpyridine (terpy) (4), or 4′-chloro-2,2′:6′,2′′-terpyridine (Cl-terpy) (5)) are described and the spectroscopic properties (both vibrational and luminescent) and stretching and interaction force constants of complexes 2, 4, and 5 are reported. Single crystal X-ray diffraction analysis of these materials shows that variation of the chelating N-donor with the same benzoic acid featuring multiple, polarizable halogens at the periphery allows for the systematic accessing of uranyl oxo atoms for non-covalent assembly, which is notable as these atoms are generally terminal. Spectroscopic characterization of complexes 2, 4, and 5 indicate that oxo atom participation in halogen bonding interactions may complement the effects of the electron donating ability of the capping ligand on corresponding uranyl luminescence and vibrational spectra, each contributing to the observed bathochromic shifts.

 Please wait while we load your content...
Please wait while we load your content...