Anisotropic Li+ ion conductivity in a large single crystal of a Co(iii) coordination complex†
Abstract
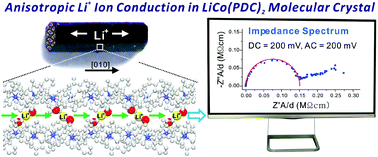
Large single crystals of a novel lithium cobalt coordination compound, LiCo(NC5H3(CO2)2)2(H2O)2.125 [LiCo(PDC)2] were grown via a hydrothermal reaction in high yield. The electrochemical impedance spectroscopy (EIS) data measured on a large single crystal of LiCo(PDC)2 revealed very interesting anisotropic Li+ ion conductivity. The redox potential for Co3+/Co4+ observed at ca. 200 mV in the cyclic voltammogram was consistent with the electric potential where the ionic conductivity occurred. Detailed structural analysis on a series of stoichiometrically equivalent cobalt coordination compounds, ACo(PDC)2 (A = Na+, K+, and H3O+), indicated that the presence of ion channels as well as a suitable cation size is critical for the anisotropic ionic conductivity.
- This article is part of the themed collection: HOT articles in Inorganic Chemistry Frontiers for 2016


 Please wait while we load your content...
Please wait while we load your content...