G-quadruplex DNA targeted metal complexes acting as potential anticancer drugs
Abstract
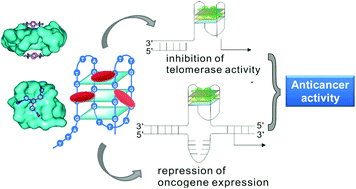
Although cisplatin and its analogues have been widely utilized as anticancer metallodrugs in clinics, their serious side effects and damage to normal tissues cannot be avoided because cisplatin kills cancer cells by attacking genomic DNA. Thus the design of metallodrugs possessing different actions of anti-cancer mechanism is promising. G-quadruplex nucleic acid, which is formed by self-assembly of guanine-rich nucleic acid sequences, has recently been considered as an attractive target for anticancer drug design. The basic unit of a G-quadruplex is a G-quartet, a planar motif generated from four guanine residues pairing together through Hoogsteen like hydrogen bonds. DNA G-quadruplex (G4) structures exist in the chromosomal telomeric sequences and the promoter regions of numerous genes, including oncogenetic promoters. Formation of G4 structures within the 3′-overhang of telomeric DNA can inhibit the telomerase activity, which is silent in normal cells but up-regulated in most cancer cells, thus significantly shortening telomeres and preventing cancer cell proliferation and immortalization. Intramolecular G4 structures formed within the oncogene promoter regions can effectively inhibit oncogenen transcription and expression. Thus rational design of small molecular ligands to selectively interact, stabilize or cleave G4 structures is a promising strategy for developing potent anti-cancer drugs with selective toxicity towards cancer cells over normal ones. This review will highlight the recent development of G4-interacting metal complexes, termed G4-ligands, discussing their binding modes with G-quadruplex DNA and their potential to serve as anticancer drugs in the medical field.
The collaboration between Prof. Zong-Wan Mao from Sun Yat-Sen University, P. R. China and Prof. Roland K. O. Sigel from the University of Zurich, Switzerland officially began in January, 2014. The international collaborative research project titled “Chemical Biology Research of New Metallodrugs for Cancer Therapy” is supported by the Science and Technology Program of Guangdong Provincial Government [20130501c]. With the rapid development of tumor molecular pharmacology, molecular targeted anti-tumor drugs have become a hot spot in the research of cancer therapy. This international collaborative research project combines the computer simulation and in vitro drug screening platform to design a series of metallodrugs that are systematic and have structural diversity, which can target specific nucleic acid structures (e.g. G-quadruplexes), key proteins (DNA topoisomerase, telomerase, CDK kinase) associated with the occurrence and development of tumor. With the advantages of both laboratories, the structural–functional relationship, interaction modes, co-crystallization, and mechanisms of action of these newly designed metallodrugs are intensively studied, and their in vitro and in vivo anti-tumor activities are comprehensively evaluated.
- This article is part of the themed collections: Inorganic Chemistry Frontiers Outstanding Paper Awards 2014-2023, 2017 Inorganic Chemistry Frontiers Review-type Articles, 2016 Inorganic Chemistry Frontiers Review-type Articles and Sino-European Collaborators

 Please wait while we load your content...
Please wait while we load your content...