C–ON bond homolysis of alkoxyamines triggered by paramagnetic copper(ii) salts†
Abstract
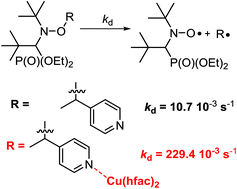
The metal complexation reactions of bis(hexafluoroacetylacetonato)copper(II) (Cu(hfac)2) with alkoxyamines (diethyl(2,2-dimethyl-1-(tert-butyl-(1-(pyridine-4-yl)ethoxy)amino)propyl)phosphonate and diethyl (2,2-dimethyl-1-(tert-butyl-(1-(pyridine-2-yl)ethoxy)amino)propyl)phosphonate) were studied. According to X-ray analysis, the molecular and crystal structures of 1 : 1 complexes depend on the configuration of the free alkoxyamines, that is dimeric (RSSR) and chain-polymeric (RR/SS) structures for para-pyridyl-substituted alkoxyamines, and cyclic unimeric (RS/SR) structure for ortho-pyridyl derivative. The complex (2 : 1 ratio Cu(hfac)2/alkoxyamine) for ortho-pyridyl-substituted alkoxyamine is not resolved. Upon warming, ortho complexes decomposed into free alkoxyamines and only a weak activation was observed. Upon warming, para complexes decomposed into their corresponding unimers, and then, a 21-fold increase in the rate constant of the C–ON bond homolysis was observed compared to the corresponding free alkoxyamines. Tuning of the homolysis rate constant of the C–ON bond via addition of pyridine is also reported.


 Please wait while we load your content...
Please wait while we load your content...