High photodegradation efficiency of phenol by mixed-metal–organic frameworks†
Abstract
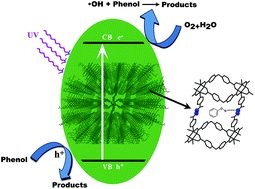
Four MOFs, [Zn(oba)(4-bpdh)0.5]n·1.5DMF (TMU-5), [Cd0.15Zn0.85(oba)(4-bpdh)0.5]n·1.5DMF (TMU-5(Cd 15%)), [Cd0.3Zn0.7(oba)(4-bpdh)0.5]n·1.5DMF (TMU-5(Cd 30%)) and [Cd(oba)(4-bpdh)]n·1DMF (TMU-7) were synthesized using non-linear dicarboxylate and linear N-donor ligands, 4,4′-oxybis(benzoic acid) (H2oba) and 2,5-bis(4-pyridyl)-3,4-diaza-2,4-hexadiene (4-bpdh), respectively, and characterized by single-crystal X-ray crystallography. These MOFs were studied for phenol degradation from aqueous solutions under UV and/or visible light irradiation without auxiliary oxidants such as H2O2. Degradation efficiency in the presence of Zn-based MOFs is higher than that of the Cd-based MOF (TMU-7). Moreover, the high percentage degradation of phenol in the presence of TMU-5(30% Cd) is largely greater than that in other studies on MOF-5 and Degussa P-25 TiO2. The photocatalytic degradation of phenol in solution obeys first-order reaction kinetics.

 Please wait while we load your content...
Please wait while we load your content...