A “turn on” fluorescent and chromogenic chemosensor for fluoride anion: experimental and DFT studies†
Abstract
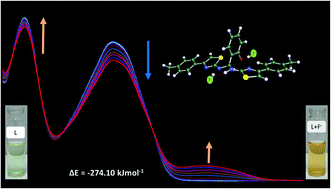
A simple, convenient “turn on” fluorescent and chromogenic chemosensor for fluoride anion detection in acetonitrile as well as in water has been developed. This probe shows high selectivity and good sensitivity towards fluoride ions. The fluoride-triggered phenolic O–H bond cleavage and hydrogen bonding between the NH group have been mechanistically investigated on the basis of spectral titration with TBAOH, 1H NMR and density functional theory (DFT) studies. The colorimetric sensing of 2-(bis((4-(p-tolyl)thiazole-2-yl)amino)methyl)phenol (BTA) was successfully utilized in the preparation of solid support silica for real sample analysis to detect F− ions. The detection limit for F− was found to be 4.1 × 10−7 M and the binding stoichiometry was proposed to be 1 : 2 based on the Job's plot and 1H NMR spectroscopic techniques.

 Please wait while we load your content...
Please wait while we load your content...