Binary nickel–iron nitride nanoarrays as bifunctional electrocatalysts for overall water splitting†
Abstract
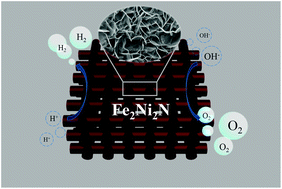
Electrochemical water splitting provides a facile method for high-purity hydrogen production, but electro-catalysts with a stable bifunctional activity towards both oxygen and hydrogen evolution have been rarely developed. Herein we report a Fe2Ni2N material with a vertically aligned nanoplate array architecture as a bifunctional catalyst for overall water splitting in an alkaline environment. This advanced catalyst affords small onset overpotentials and fast current density increase, resulting in an excellent water splitting performance (requiring 1.65 V for achieving 10 mA cm−2), superior to the combination of benchmark noble metal catalysts.
- This article is part of the themed collection: 2015 Emerging Investigators by ICF

 Please wait while we load your content...
Please wait while we load your content...