A high-spin nickel(ii) borohydride complex in dehalogenation†
Abstract
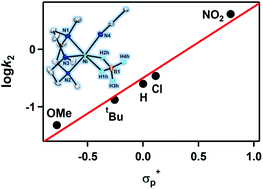
A nickel(II)–borohydride complex bearing a macrocyclic tridentate N-donor ligand, [Ni(Me3-TACN)(BH4)(CH3CN)]+ (Me3-TACN = 1,4,7-trimethyl-1,4,7-triazacyclononane), was prepared, isolated, and characterized by various physicochemical methods, including UV-vis, ESI-MS, IR and X-ray analyses. The structural and spectroscopic characterization clearly shows that the borohydride ligand is bound to the high-spin nickel(II) center in an η2-manner. Density functional theory calculations provided geometric information of 2, showing that the η2-binding of borohydride to the nickel center is more favorable than the η3-binding mode in CH3CN. The complex is paramagnetic with an effective magnetic moment of 2.9μB consistent with a d8 high-spin system. The reactivity of the high-spin nickel(II)–borohydride complex was examined in dehalogenation with numerous halocarbons. A kinetic isotope effect value of 1.7 was observed in the dehalogenation of CHCl3 by the nickel(II)–borohydride complex. Kinetic studies and isotopic labeling experiments implicate that hydride ion or hydrogen atom transfer from the borohydride group is the rate determining step. The positive Hammett ρ value of 1.2, obtained in the reactions of [Ni(Me3-TACN)(BH4)(CH3CN)]+ and para-substituted benzoyl chloride, indicates that the dehalogenation by the nickel(II)–borohydride species occurs via a nucleophilic reaction.
- This article is part of the themed collection: 2015 Emerging Investigators by ICF

 Please wait while we load your content...
Please wait while we load your content...