Conventional fluorophore-free dual pH- and thermo-responsive luminescent alternating copolymer†
Abstract
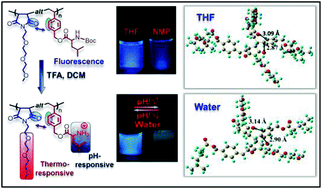
Herein, we report the synthesis of a new class of traditional fluorophore-free dual pH- and thermo-responsive fluorescent copolymer through sequence-controlled copolymerization of rationally designed monomers. The N-substituted maleimide monomer bearing a diethylene oxide side-chain, (N-(methoxy diethylene glycol) maleimide, M1), was copolymerized with a tert-butyl carbamate (Boc)-protected leucine appended styrenic monomer (M2) to obtain well-defined copolymers with perfectly alternating sequences of M1 and M2. The as-synthesized copolymers displayed bright-blue fluorescence in organic solvents. After Boc-group expulsion, the copolymers showed dual pH- and thermo-responsiveness, they retained their luminescence properties in organic solvents, and also showed pH/thermo-tunable fluorescence activity in water. The origin of the fluorescence in the copolymers was ascertained using density functional theory (DFT), where we observed that the “through-space” π–π interaction between the benzene ring and the neighbouring carbonyl group of the maleimide unit is responsible for the unexpected fluorescence in the alternating copolymer.
- This article is part of the themed collection: Advisory Board’s top picks from Polymer Chemistry

 Please wait while we load your content...
Please wait while we load your content...