Poly(thioacrylate)s: expanding the monomer toolbox of functional polymers†
Abstract
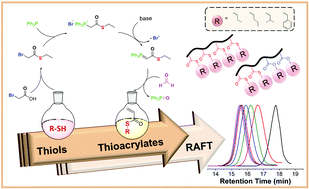
Radically polymerizable monomers such as acrylates, acrylamides, and styrenes have been investigated because of their corresponding polymers for many years. However, thioacrylates are a forgotten class of monomers and have not been studied for decades. Herein, we present a simple synthetic approach to prepare various thioacrylate monomers as well as their controlled radical polymerization for the first time. Four different thioacrylate (TA) monomers namely, ethyl thioacrylate, phenyl thioacrylate, n-propyl thioacrylate and isopropyl thioacrylate have been polymerized via RAFT polymerization. A trithiocarbonate chain transfer agent was utilized to control the molar mass and dispersity of thioacrylate polymers. Homopolymers of thioacrylates with different degrees of polymerizations were prepared and their block copolymerizations were studied with ethylacrylate and thioacrylates. All obtained polymers were analyzed in detail using GPC, NMR, TGA, DSC and water contact angle.


 Please wait while we load your content...
Please wait while we load your content...