Copolymerization of alkyl diazoacetates with α,β-unsaturated aldehydes: synthesis and application†
Abstract
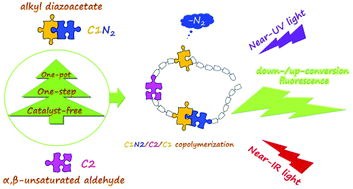
Copolymerization between alkyl diazoacetates and α,β-unsaturated aldehydes, which is a one-pot, one-step and catalyst-free reaction, is presented. The product is a ternary copolymer composed of an alkyl diazoacetate, an α,β-unsaturated aldehyde and an α-carbalkoxy carbene, which is derived from the alkyl diazoacetate by the release of N2. Thus, the reaction is named as the C1N2/C2/C1 copolymerization. Various diazoacetates and α,β-unsaturated aldehydes were utilized to establish the methodology. The products are down-/up-conversion fluorescence molecules that can significantly enhance cell imaging through the synchronized excitation of near-ultraviolet and near-infrared light and may be widely used in other optical fields. The up-conversion fluorescence is the result of a two-photon excitation process.


 Please wait while we load your content...
Please wait while we load your content...