Poly(3-imidazolyl-2-hydroxypropyl methacrylate) – a new polymer with a tunable upper critical solution temperature in water
Abstract
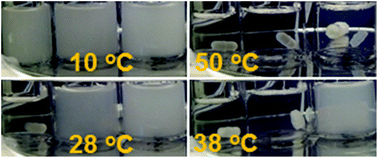
Opening the epoxide rings of poly(glycidyl methacrylate) (PGMA) with imidazole produces poly(3-imidazolyl-2-hydroxypropyl methacrylate) (PiGMA). PiGMA is soluble in room-temperature water due to protonation of the unsubstituted imidazole nitrogen atom at pH values below 5.9 but becomes insoluble at pH values above 6.5 due to deprotonation. At pH values between 6.2 and 6.5, partially protonated PiGMA is insoluble in water at low temperatures but becomes soluble upon heating. The transmittance variation of aqueous PiGMA solutions at 500 nm has been measured and used to monitor the dissolution or aggregation of PiGMA samples. The cloud temperature Tc determined from these transmittance curves increases at pH = 6.30 from 23.2 ± 0.3 to 93.2 ± 0.3 °C as the repeat unit number of PiGMA increases from 15 to 140. Increasing the solution pH from 6.2 to 6.5 for a PiGMA sample with 50 repeat units increases its Tc from 20.4 ± 0.6 to 89.7 ± 0.5 °C. Since PGMA can be readily synthesized and the reaction between imidazole and PGMA occurs under mild conditions, this paper offers a ready recipe for a family of pH- and thermo-responsive polymers.

 Please wait while we load your content...
Please wait while we load your content...