Synthesis and electrochemical polymerization of diketopyrrolopyrrole based donor–acceptor–donor monomers containing 3,6- and 2,7-linked carbazoles†
Abstract
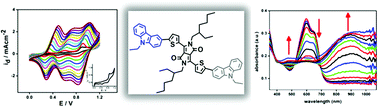
Three new donor–acceptor–donor (D–A–D) type monomers bearing 2,7- or 3,6-linked carbazoles as the donor unit and 2,5-dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione (DPP) as the electron poor acceptor unit were synthesized via a Suzuki cross coupling reaction. The monomers were electrochemically polymerized both by repetitive cycling and by constant potential electrolysis in acetonitrile–tetrabutylammonium hexafluorophosphate electrolytic medium containing boron trifluoride diethyl etherate (BFEE). Optical and electrochemical properties of the monomers and their corresponding polymers were investigated and it was found that optical properties show slight variations depending on both the substituent on the nitrogen atom and the linkage site. Monomers, besides reversible oxidations and reversible reduction peaks similar to those present in the differential pulse voltammogram of the acceptor unit, exhibit one irreversible oxidation peak responsible for initiating the electrochemical polymerization of the monomers. Both electrochemical and optical data were used to elucidate the band gap (Eg) values of the polymers and it was found that polymer films obtained from 3,6-linked carbazole derivatives have slightly lower Eg values compared to 2,7-linked derivatives. Furthermore, based on the observed electrochemical behavior of the monomers, a possible mechanism for the electrochemical oxidation was proposed.

 Please wait while we load your content...
Please wait while we load your content...