Multicomponent polymerization: development of a one-pot synthetic route to functional polymers using diyne, N-sulfonyl azide and water/ethanol as reactants†
Abstract
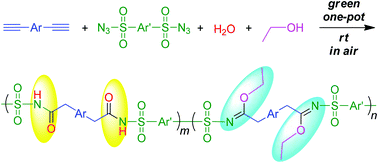
Green chemistry shows the advantages of a simple synthetic process, low cost of production, a clean working environment and reduction of harmful wastes. In this work, we used naturally occurring materials to prepare functional polymers. The polymerizations of 1,2-bis(4-ethynylphenyl)-1,2-diphenylethene, disulfonyl azide, and water together with ethanol were catalyzed by copper(I) iodide and triethylamine in a mild, efficient and atom-economical fashion, generating soluble polymers with high molecular weights in satisfactory isolation yields. By controlling the adding amount of water and ethanol, the ratio of amide and imidate units in the polymer chain could be readily modulated. Their good solubility allows the polymers to show outstanding film-forming ability. Due to the tetraphenylethene moieties embedded in the polymer chains, the polymer solutions emit faintly, but their aggregates fluoresce intensely, demonstrating a characteristic of aggregation-induced emission. Such results are anticipated to accelerate the development of efficient and economical one-pot multicomponent polymerizations toward functional polymeric materials.

 Please wait while we load your content...
Please wait while we load your content...