Trityl-based alkoxyamines as NMP controllers and spin-labels†
Abstract
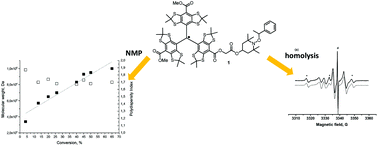
Recently, new applications of trityl-nitroxide biradicals were proposed. In the present study, attachment of a trityl radical to alkoxyamines was performed for the first time. The rate constants kd of C–ON bond homolysis in these alkoxyamines were measured and found to be similar to those for alkoxyamines without a trityl moiety. The electron paramagnetic resonance (EPR) spectra of the products of alkoxyamine homolysis (trityl-TEMPO and trityl-SG1 biradicals) were recorded, and the corresponding exchange interactions were estimated. The decomposition of trityl-alkoxyamines showed more than an 80% yield of biradicals, meaning that the C–ON bond homolysis is the main reaction. The suitability of these labelled initiators/controllers for polymerisation was exemplified by means of a successful nitroxide-mediated polymerisation (NMP) of styrene. Thus, this is the first report of a spin-labelled alkoxyamine suitable for NMP.


 Please wait while we load your content...
Please wait while we load your content...