Stimulus-responsive non-ionic diblock copolymers: protonation of a tertiary amine end-group induces vesicle-to-worm or vesicle-to-sphere transitions†
Abstract
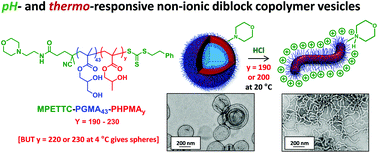
A well-defined poly(glycerol monomethacrylate) (PGMA) macromolecular chain transfer agent (macro-CTA) with a mean degree of polymerisation (DP) of 43 was prepared by reversible addition–fragmentation chain transfer (RAFT) polymerisation using a morpholine-functionalised trithiocarbonate-based chain transfer agent (MPETTC). Chain extension of this macro-CTA by RAFT aqueous dispersion polymerisation of 2-hydroxypropyl methacrylate (HPMA) at pH 7.0–7.5 produced a series of four MPETTC-PGMA43-PHPMAy vesicles (where y = 190, 200, 220 or 230). Protonation of the morpholine end-group increases the hydrophilic character of the PGMA stabiliser block, which leads to a reduction in the packing parameter for the diblock copolymer chains. However, such pH-responsive behaviour critically depends on the value of y. For y = 190 or 200, lowering the solution pH to pH 3 induces a vesicle-to-worm transition at 20 °C according to dynamic light scattering, aqueous electrophoresis, transmission electron microscopy and turbidimetry studies. This order–order transition is suppressed in the presence of added electrolyte, which screens the cationic end-groups. In addition, no change in copolymer morphology was observed on lowering the solution temperature at neutral pH, regardless of the y value. The diblock copolymer nano-objects obtained at pH 3 were also cooled to 4 °C to examine their dual stimulus-responsive behaviour to both pH and temperature triggers. In all four cases, a change in morphology from either worms or vesicles to afford spheres (or spheres plus relatively short worms) was observed. Temperature-dependent oscillatory rheology experiments performed on cationic worms at pH 3 indicated a worm-to-sphere transition on cooling from 20 °C to 4 °C, which leads to reversible degelation. In summary, spheres, worms or vesicles can be obtained for MPETTC-PGMA-PHPMA diblock copolymers on first lowering the solution pH to pH 3, followed by cooling from 20 °C to 4 °C.
- This article is part of the themed collections: Stimulus-responsive polymers and Open access articles from Polymer Chemistry

 Please wait while we load your content...
Please wait while we load your content...