Vinyl monomers bearing a sulfonyl(trifluoromethane sulfonyl) imide group: synthesis and polymerization using nitroxide-mediated polymerization†
Abstract
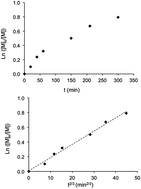
A series of water-soluble styrene and propyl (meth)acrylate based monomers bearing a sulfonyl(trifluoromethane sulfonyl) imide (STFSI) group was prepared. These monomers were synthesized in good yield from the corresponding chloride sulfonyl monomer and trifluoromethanesulfonamide. Their chemical structures were further confirmed by HR-MS and NMR spectroscopy. Nitroxide-mediated polymerization of the prepared monomers was then performed in aqueous solution at 100 °C, 90 °C and 75 °C for acrylate, styrene and methacrylate derivatives, respectively. Controlled polymerization was successfully achieved as illustrated by the linearity of ln([M]0/[M]) vs. t2/3, the molar mass increase with conversion and the relatively low dispersity values for the resulting homopolymers. Compared to their neutral analogous polymers, the prepared anionic homopolymers exhibited a higher glass-transition temperature. This phenomenon was attributed to the electrostatic interactions between STFSI side groups along the polymer backbone.

 Please wait while we load your content...
Please wait while we load your content...