D–D–A dyes with phenothiazine–carbazole/triphenylamine as double donors in photopolymerization under 455 nm and 532 nm laser beams†
Abstract
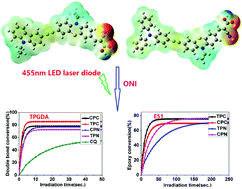
In order to enhance electron-donating ability and absorptivity in the visible region of the spectrum, four novel organic dyes (CPC, TPC, CPN and TPN) with carbazole–phenothiazine or triphenylamine–phenothiazine as double electron donors (D), and cyanoacrylic acid or an aldehyde group as the electron acceptor (A) have been designed and synthesized. The dyes exhibit charge transfer properties in the ground and excited states and absorb strongly in the spectral range of 380–550 nm. Upon exposure to irradiation of a laser diode at 455 and 532 nm, the dyes together with an iodonium salt induced high polymerization efficiency in the cationic polymerization of bisphenol-A epoxy resin A (DGEBA) and free radical polymerization of tripropylene glycol diacrylate (TPGDA). An electron-transfer photosensitization mechanism was established based on steady-state photolysis experiments, theoretical calculations of molecular orbitals, and electrochemical analysis. Our results showed that these dyes would have extensive application in photopolymerization promoted by soft visible light.

 Please wait while we load your content...
Please wait while we load your content...