Protein–polymer conjugates prepared via host–guest interactions: effects of the conjugation site, polymer type and molecular weight on protein activity†
Abstract
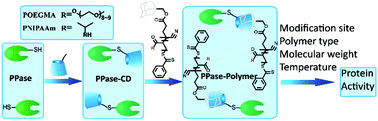
Generating well-defined protein–polymer conjugates and fully understanding their properties can efficiently promote the development of protein therapeutics. To maintain and control the protein activity under different conditions is important for the application of protein–polymer conjugates. Herein, maleimido-functionalized cyclodextrin was conjugated to model proteins, mutational-introduced site-specific thiol-functional inorganic pyrophosphatase (PPase). The cyclodextrin-functionalized PPase was then reacted with adamantyl-functionalized poly(N-isopropyl acrylamide) (Ada-PNIPAAm) or poly(oligo(ethylene glycol)methyl ether acrylate) (Ada-POEGMA) to generate well-defined protein–polymer conjugates via the mild and facile host–guest chemistry. The conjugates were confirmed by gel permeation chromatography (GPC) and high-performance liquid chromatography (HPLC), and the properties of the conjugates with variable conjugation sites, polymer type, and molecular weight were then investigated in detail. A suitable conjugation site and a certain molecular weight are needed to guarantee the best activity-keeping property at different temperatures.

 Please wait while we load your content...
Please wait while we load your content...