Synthesis, characterisation and Pickering emulsifier performance of poly(stearyl methacrylate)–poly(N-2-(methacryloyloxy)ethyl pyrrolidone) diblock copolymer nano-objects via RAFT dispersion polymerisation in n-dodecane†
Abstract
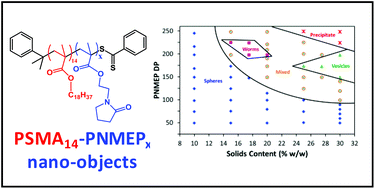
A near-monodisperse poly(stearyl methacrylate) macromolecular chain transfer agent (PSMA macro-CTA) was prepared via reversible addition–fragmentation chain transfer (RAFT) solution polymerisation in toluene. This PSMA macro-CTA was then utilised as a stabiliser block for the RAFT dispersion polymerisation of a highly polar monomer, N-2-(methacryloyloxy)ethyl pyrrolidone (NMEP), in n-dodecane at 90 °C. 1H NMR studies confirmed that the rate of NMEP polymerisation was significantly faster than that of a non-polar monomer (benzyl methacrylate, BzMA) under the same conditions. For example, when targeting a PSMA14–PNMEP100 diblock copolymer, more than 99% NMEP conversion was achieved within 30 min, whereas only 19% BzMA conversion was obtained on the same time scale for the corresponding PSMA14–PBzMA100 synthesis. The resulting PSMA–PNMEP diblock copolymer chains underwent polymerisation-induced self-assembly (PISA) during growth of the insoluble PNMEP block to form either spherical micelles, highly anisotropic worms or polydisperse vesicles, depending on the target DP of the PNMEP chains. Systematic variation of this latter parameter, along with the solids content, allowed the construction of a phase diagram which enabled pure morphologies to be reproducibly targeted. Syntheses conducted at 10% w/w solids led to the formation of kinetically-trapped spheres. A monotonic increase in particle diameter with PNMEP DP was observed for such PISA syntheses, with particle diameters of up to 462 nm being obtained for PSMA14–PNMEP960. Increasing the copolymer concentration to 15% w/w solids led to worm-like micelles, while vesicles were obtained at 27.5% w/w solids. High (≥95%) NMEP conversions were achieved in all cases and 3 : 1 chloroform/methanol GPC analysis indicated relatively high blocking efficiencies. However, relatively broad molecular weight distributions (Mw/Mn > 1.50) were observed when targeting PNMEP DPs greater than 150. This indicates light branching caused by the presence of a low level of dimethacrylate impurity. Finally, PSMA14–PNMEP49 spheres were evaluated as Pickering emulsifiers. Unexpectedly, it was found that either water-in-oil or oil-in-water Pickering emulsions could be obtained depending on the shear rate employed for homogenisation. Further investigation suggested that high shear rates lead to in situ inversion of the initial hydrophobic PSMA14–PNMEP49 spheres to form hydrophilic PNMEP49–PSMA14 spheres.
- This article is part of the themed collections: Advisory Board’s top picks from Polymer Chemistry and Open access articles from Polymer Chemistry

 Please wait while we load your content...
Please wait while we load your content...