The synthesis and tissue adhesiveness of temperature-sensitive hyperbranched poly(amino acid)s with functional side groups†
Abstract
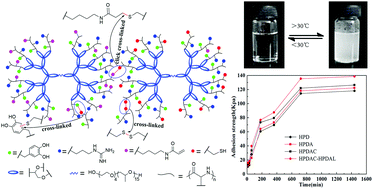
The incorporation of L-3,4-dihydroxyphenylalanine (L-DOPA) residues in mussel-like biomimetic copolymers has drawn great attention due to their adhesive properties. In this work, a series of temperature-sensitive and hyperbranched poly(amino acid)s containing different functional side groups (catechol, guanidyl, mercapto and double bond) were designed and synthesized by the ring-opening polymerization of N-carboxy-α-amino acid anhydride (NCA), using a temperature-sensitive and multi-hydroxyl end pluronic L-31 as the initiator in this process. The tissue adhesive properties of these copolymers were evaluated by tensile strength tests on wet pork skin. We found that the topological structure, side groups of copolymers, adhesive temperature and cure time had influence on the wet adhesive strength, especially side groups. While only L-DOPA was incorporated into copolymeric chains, the wet adhesive strength was about 106 kPa. With guanidyl, mercapto and double bonds incorporated into copolymeric chains, an obvious increase in the wet adhesive strength was observed. Additionally, the wet adhesive strength improved by about 20 kPa at body temperature (37 °C) as compared to that at room temperature (25 °C). Meanwhile, we found that it has good biocompatibility and degradability through its cytotoxicity and degradation tests.

 Please wait while we load your content...
Please wait while we load your content...