Synthesis and hydrolytic properties of water-soluble poly(carbonate–hydroxyurethane)s from trimethylolpropane†
Abstract
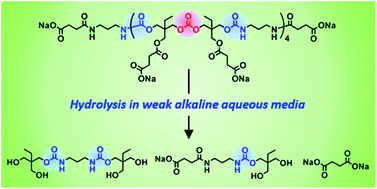
Water-soluble poly(carbonate–hydroxyurethane)s (PCHUs) comprising hydroxyurethane–carbonate–hydroxyurethane repeating units with well-controlled molecular weight and polydispersity were synthesized from trimethylolpropane (TMP) and investigated for their hydrolytic properties in aqueous media at different pH values. The reaction of TMP with diphenyl carbonate affords mainly three types of different-structured TMP-based carbonates. By fine tuning the synthetic conditions, the bifunctional six-membered cyclic carbonate bridged by an acyclic carbonate bond (TMPC1) was obtained in 36.8% yield after purification. The polyaddition of TMPC1 and 1.25 equiv. of 1,3-diaminopropane at −10 °C proceeds without the cleavage of the acyclic carbonate linkages to form PCHUs comprising four repeating units with amino terminals. Furthermore, the reaction of their amino terminals with α,α′-diisothiocyante-p-xylene forms thiourea linkages (–NH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–NH–) to extend the chain length of the PCHUs without the inter/intra chain cross-linking via their hydroxyl side chains. After carboxylation of the hydroxyl side chains of the PCHUs with succinic anhydride and neutralization with sodium bicarbonate, water-soluble PCHUs can be obtained. The urethane bonds of the PCHUs are stable, while carbonate and ester bonds are gradually hydrolyzed in alkaline aqueous media at ambient temperature. In particular, the water-soluble PCHUs are completely decomposed to their basic structures in carbonate buffers at pH 10.6 within one week.
S)–NH–) to extend the chain length of the PCHUs without the inter/intra chain cross-linking via their hydroxyl side chains. After carboxylation of the hydroxyl side chains of the PCHUs with succinic anhydride and neutralization with sodium bicarbonate, water-soluble PCHUs can be obtained. The urethane bonds of the PCHUs are stable, while carbonate and ester bonds are gradually hydrolyzed in alkaline aqueous media at ambient temperature. In particular, the water-soluble PCHUs are completely decomposed to their basic structures in carbonate buffers at pH 10.6 within one week.

 Please wait while we load your content...
Please wait while we load your content...