Post-polymerisation modification of bio-derived unsaturated polyester resins via Michael additions of 1,3-dicarbonyls†
Abstract
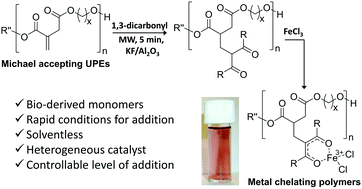
Post-polymerisation modification of α,β-unsaturated polyesters (UPEs) is useful to deliver polymers with tuneable properties and applications different from their parent backbone. Bio-derivable itaconate unsaturated polyesters, with a range of co-monomers, were modified via a heterogeneously catalysed microwave-assisted Michael addition of pendants, acetylacetone (Hacac) and dimethyl malonate (DMM), to the polymer backbones with very short reaction times. Differential scanning calorimetry analysis showed an increase in the glass-transition temperatures of most of the saturated polyesters considered. Solubility and complexation studies demonstrated metal chelating abilities of the acetylacetone pendant can be retained, even following tethering to a polyester backbone. Additionally, it is demonstrated for the first time that Michael addition with Hacac and DMM can be used to reverse Ordelt saturation, an unwanted side-reaction in the synthesis of UPEs.

 Please wait while we load your content...
Please wait while we load your content...