Intermolecular hydrogen bonding controlled stereoselective photocycloaddition of vinyl ethers to 1-cyanonaphthalenes†
Abstract
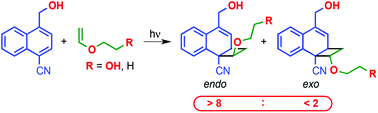
With the aim of developing photoreactions that use intermolecular hydrogen bonding interactions to control the efficiency and stereochemistry, the 1,2-[2 + 2] photocycloaddition reactions of 1-cyanonaphthalene derivatives with vinyl ethers possessing hydroxy groups were examined. The photoreaction of 1-cyano-4-(hydroxymethyl)naphthalene (1a) with 2-hydroxyethyl vinyl ether (2a) at room temperature was found to produce cycloadducts, endo-3aa and exo-3aa, in a non-stereoselective manner (56 : 44). However, this photoreaction at −40 °C displays a high endo-selectivity (81 : 19). In the reaction of 1a with ethyl vinyl ether (2b), high endo selectivity was observed both at room temperature and at −40 °C. The endo selectivity in the [2 + 2] photocycloaddition process is attributed to the intermolecular hydrogen bonding interactions between the reacting partners in the ground and excited states. Evidence to support this conclusion comes from the results of VT NMR and fluorescence quenching experiments, as well as the photoreactions of deuterated substrates.


 Please wait while we load your content...
Please wait while we load your content...